Clinicaltrials gov search by nct number.
If you’re looking for clinicaltrials gov search by nct number images information connected with to the clinicaltrials gov search by nct number topic, you have pay a visit to the ideal site. Our website frequently gives you suggestions for seeking the highest quality video and image content, please kindly search and locate more informative video content and images that fit your interests.
 Clinicaltrials Gov Nlm Musings From The Mezzanine From nlmdirector.nlm.nih.gov
Clinicaltrials Gov Nlm Musings From The Mezzanine From nlmdirector.nlm.nih.gov
This number is listed prominently on each specific studys page and is always preceded by the letters NCT. NCT Number Notice of Noncompliance Close Out Letter if any Civil Money Penalty Amount if. Search FDA Submit search. Search Tips and Examples.
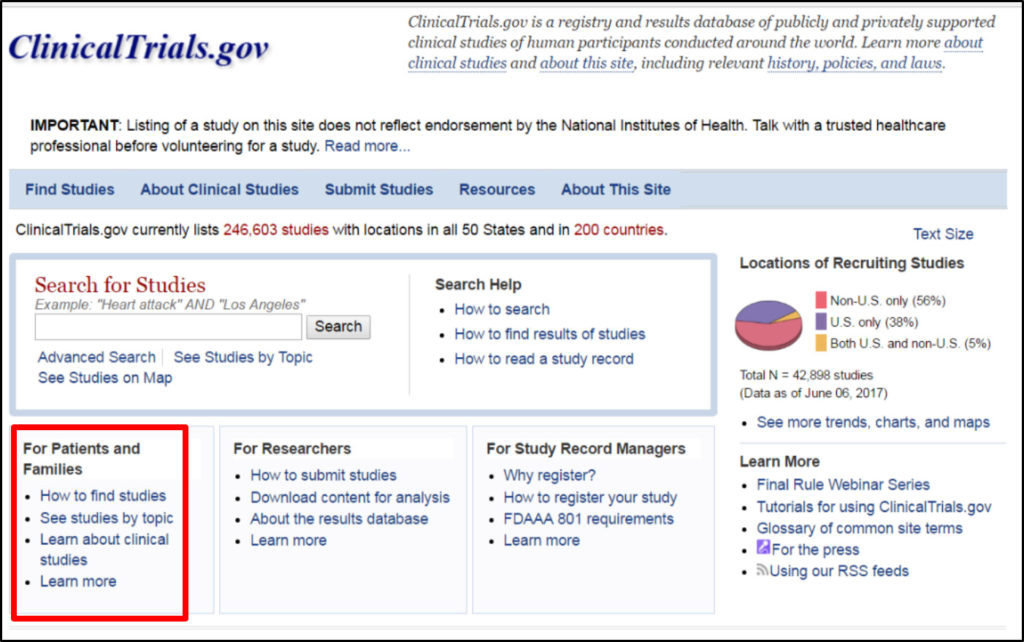
Use AND in uppercase to search for multiple terms.
I completed a clinical trial that studied an investigational product drug biological product or device that is not initially approved licensed or cleared by the FDA. The most common reasons why an NCT number has not yet been assigned include. ClinicalTrialsgov - Notices of. It is planned to recruit 111 subjects for 2 vaccines Eru-CoV-Vac or CoronaVac. ClinicalTrialsgov Registration Data Elements 2 1.
 Source: cllsociety.org
Source: cllsociety.org
DFHCC requires an NCT number prior to activation. It is planned to recruit 111 subjects for 2 vaccines Eru-CoV-Vac or CoronaVac. The NCT Number also called the ClinicalTrialsgov Identifier is assigned after the protocol information has been Released submitted by the Responsible Party and passed review by ClinicalTrialsgov staff. Until an NCT number is assigned the study is not registered. Janssen Research Development LLC First Posted.
At that time an e-mail notification containing the NCT Number is sent.
Clicking the title should redirect to the Study Record Detail page. ClinicalTrialsgov Registration Data Elements 2 1. Clicking the title should redirect to the Study Record Detail page. DFHCC requires an NCT number prior to activation.
 Source: slidetodoc.com
Source: slidetodoc.com
Search Tips and Examples. HMPL-306 is a dual IDH12 inhibitor. NCT04963738 Other Study ID Numbers. Likewise the ClinicalTrialsgov record must list the IRB number as the Unique Protocol ID so that the records can be linked.
 Source: prnewswire.com
Source: prnewswire.com
Secondary Identifying Numbers Unique Protocol Identification Number Secondary IDs 4. This is a phase 1 open-label multicenter single-arm study to evaluate safety tolerability PK PD and preliminary efficacy of HMPL-306 administered orally in treatment of subjects with advanced relapsed refractory or resistant hematological malignancies that harbor IDH mutations or co-mutations. The most common reasons why an NCT number has not yet been assigned include. July 15 2021 Key Record Dates.
 Source: slideplayer.com
Source: slideplayer.com
Clicking the title should redirect to the Study Record Detail page. CR109019 2021-001048-85 EudraCT Number 73763989HPB1003 Other Identifier. Click on the links below to practice some sample searches. DFHCC requires an NCT number prior to activation.
Please refer to this study by its ClinicalTrialsgov identifier NCT number. There is no intent to seek approval clearance or licensure of the product by the FDA for example development of. The NCT Number also called the ClinicalTrialsgov Identifier is assigned after the protocol information has been Released submitted by the Responsible Party and passed review by ClinicalTrialsgov staff. To determine the safety and immunogenicity of booster doses of vaccine against SARS-CoV2 after a minimum of 90 days and a maximum of 270 days after the 2nd dose of a homologous 2 dose primary regimen.
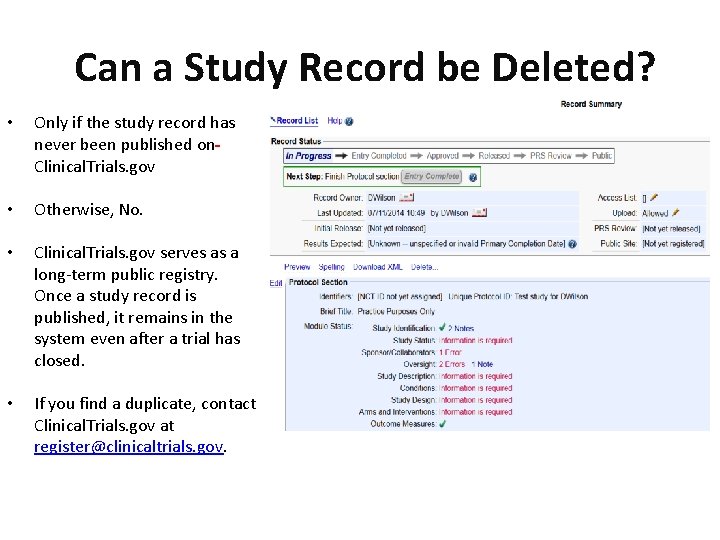
The National Clinical Trial number is an identification that ClinicalTrialsgov assigns a study when it is registered.
NCT04963738 Other Study ID Numbers. Click on the links below to practice some sample searches. Entering your NCT into Velos Search the study in Velos and Click on the More Study Details icon located next to the Study Number field. Primary Registry and Trial Identifying Number ClinicalTrialsgov Identifier NCT Number - assigned by system 2. An identifiers ID if any other than the organizations Unique Protocol Identification Number or the NCT number that is assigned to the clinical study.
 Source: slidetodoc.com
Source: slidetodoc.com
The most common reasons why an NCT number has not yet been assigned include. It is planned to recruit 111 subjects for 2 vaccines Eru-CoV-Vac or CoronaVac. Date of Registration in Primary Registry Generated by system 3. Name of the individual or entity responsible for registering the study for each study being conducted under the application. Use AND in uppercase to search for multiple terms.
CR109019 2021-001048-85 EudraCT Number 73763989HPB1003 Other Identifier. Effective January 1 2014 it will be mandatory to report the clinical trial number on claims for itemsservices provided in all clinical trials that are. To determine the safety and immunogenicity of booster doses of vaccine against SARS-CoV2 after a minimum of 90 days and a maximum of 270 days after the 2nd dose of a homologous 2 dose primary regimen. ClinicalTrialsgov - Notices of.
August 4 2021 Last Verified.
DFHCC requires an NCT number prior to activation. At that time an e-mail notification containing the NCT Number is sent. You can enter a word or a phrase such as the name of a medical condition or an intervention. An NCT number is assigned by ClinicalTrialsgov once a registration is accepted.
 Source: slidetodoc.com
Source: slidetodoc.com
It is planned to recruit 111 subjects for 2 vaccines Eru-CoV-Vac or CoronaVac. July 15 2021 Key Record Dates. Janssen Research Development LLC First Posted. This is a phase 1 open-label multicenter single-arm study to evaluate safety tolerability PK PD and preliminary efficacy of HMPL-306 administered orally in treatment of subjects with advanced relapsed refractory or resistant hematological malignancies that harbor IDH mutations or co-mutations.
 Source: prnewswire.com
Source: prnewswire.com
Effective January 1 2014 it will be mandatory to report the clinical trial number on claims for itemsservices provided in all clinical trials that are. For more information see How to Search. Until an NCT number is assigned the study is not registered. To determine the safety and immunogenicity of booster doses of vaccine against SARS-CoV2 after a minimum of 90 days and a maximum of 270 days after the 2nd dose of a homologous 2 dose primary regimen.
 Source: slidetodoc.com
Source: slidetodoc.com
To learn more about this study you or your doctor may contact the study research staff using the contact information provided by the sponsor. The NCT will be located under the Brief Title listed as the ClinicalTrialsgov Identifier. The Centers for Medicare and Medicaid Services CMS issued a Transmittal requiring new mandatory reporting of the clinicaltrialsgov clinical trial number also known as NCT on all hospital and professional claims for related itemsservices. Primary Registry and Trial Identifying Number ClinicalTrialsgov Identifier NCT Number - assigned by system 2.
The most common reasons why an NCT number has not yet been assigned include.
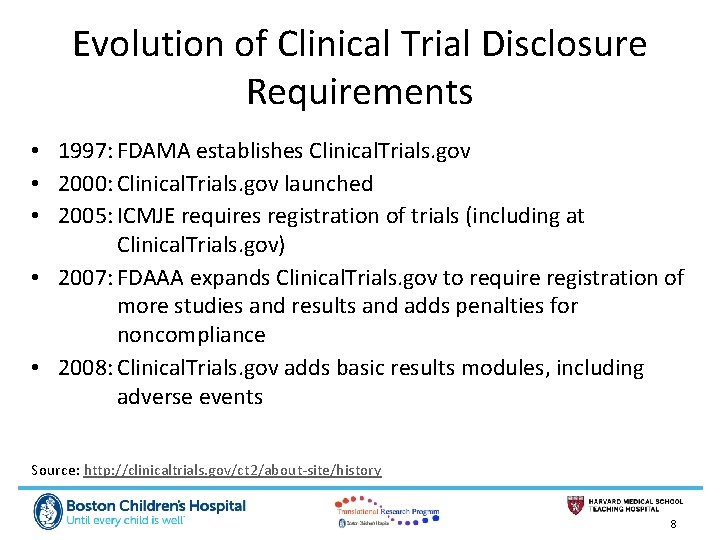
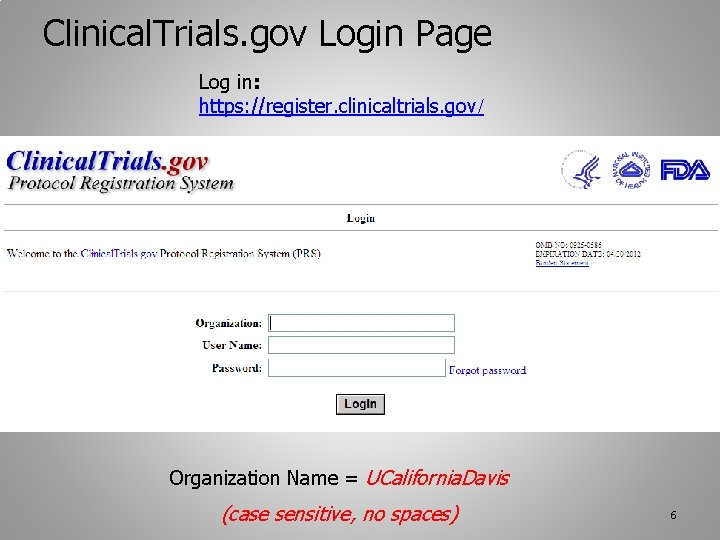
Likewise the ClinicalTrialsgov record must list the IRB number as the Unique Protocol ID so that the records can be linked. Name of the individual or entity responsible for registering the study for each study being conducted under the application. Every study on ClinicalTrialsgov is assigned a unique number called the ClinicalTrialsgov Identifier NCT Number. The National Clinical Trial number is an identification that ClinicalTrialsgov assigns a study when it is registered. The NCT number in the IRB application must be entered in VIIB1b of the IRB application prior to IRB approval to show that the ClinicalTrialsgov record has been created.
 Source: slidetodoc.com
Source: slidetodoc.com
National Clinical Trial NCT number ie. The Centers for Medicare and Medicaid Services CMS issued a Transmittal requiring new mandatory reporting of the clinicaltrialsgov clinical trial number also known as NCT on all hospital and professional claims for related itemsservices. To learn more about this study you or your doctor may contact the study research staff using the contact information provided by the sponsor. National Clinical Trial NCT Identifier Number The NCT identifier number is assigned by the National Library of Medicine NLM at httpclinicaltrialsgov website when a new study appears in the NLM Clinical Trials data base. This includes any unique clinical study identifiers assigned by other publicly available clinical trial registries.
You can enter a word or a phrase such as the name of a medical condition or an intervention.
This is a phase 1 open-label multicenter single-arm study to evaluate safety tolerability PK PD and preliminary efficacy of HMPL-306 administered orally in treatment of subjects with advanced relapsed refractory or resistant hematological malignancies that harbor IDH mutations or co-mutations. Every study on ClinicalTrialsgov is assigned a unique number called the ClinicalTrialsgov Identifier NCT Number. National Clinical Trial NCT Identifier Number The NCT identifier number is assigned by the National Library of Medicine NLM at httpclinicaltrialsgov website when a new study appears in the NLM Clinical Trials data base. To determine the safety and immunogenicity of booster doses of vaccine against SARS-CoV2 after a minimum of 90 days and a maximum of 270 days after the 2nd dose of a homologous 2 dose primary regimen.
 Source: slidetodoc.com
Source: slidetodoc.com
2 days agoPhase 2. The record has not been approved and released by the responsible party typically the sponsor-investigator. To learn more about this study you or your doctor may contact the study research staff using the contact information provided by the sponsor. The NCT number is in the format NCTXXXXXXXX.
 Source: www1.udel.edu
Source: www1.udel.edu
The ClinicalTrialsgov number Brief Title as listed in ClinicalTrialsgov and. The National Clinical Trial number is an identification that ClinicalTrialsgov assigns a study when it is registered. The NCT number is in the format NCTXXXXXXXX. For more information see How to Search.
 Source: cllsociety.org
Source: cllsociety.org
To learn more about this study you or your doctor may contact the study research staff using the contact information provided by the sponsor. I completed a clinical trial that studied an investigational product drug biological product or device that is not initially approved licensed or cleared by the FDA. July 15 2021 Key Record Dates. The Centers for Medicare and Medicaid Services CMS issued a Transmittal requiring new mandatory reporting of the clinicaltrialsgov clinical trial number also known as NCT on all hospital and professional claims for related itemsservices.
Search FDA Submit search.
ClinicalTrialsgov Registration Data Elements 2 1. Date of Registration in Primary Registry Generated by system 3. CR109019 2021-001048-85 EudraCT Number 73763989HPB1003 Other Identifier. The NCT Number also called the ClinicalTrialsgov Identifier is assigned after the protocol information has been Released submitted by the Responsible Party and passed review by ClinicalTrialsgov staff. Primary Registry and Trial Identifying Number ClinicalTrialsgov Identifier NCT Number - assigned by system 2.
 Source: slideplayer.com
Source: slideplayer.com
August 4 2021 Last Verified. National Clinical Trial NCT Identifier Number The NCT identifier number is assigned by the National Library of Medicine NLM at httpclinicaltrialsgov website when a new study appears in the NLM Clinical Trials data base. This is a phase 1 open-label multicenter single-arm study to evaluate safety tolerability PK PD and preliminary efficacy of HMPL-306 administered orally in treatment of subjects with advanced relapsed refractory or resistant hematological malignancies that harbor IDH mutations or co-mutations. CR109019 2021-001048-85 EudraCT Number 73763989HPB1003 Other Identifier. For more information see How to Search.
National Clinical Trial NCT Identifier Number The NCT identifier number is assigned by the National Library of Medicine NLM at httpclinicaltrialsgov website when a new study appears in the NLM Clinical Trials data base.
NCT04963738 Other Study ID Numbers. Clicking the title should redirect to the Study Record Detail page. Primary Registry and Trial Identifying Number ClinicalTrialsgov Identifier NCT Number - assigned by system 2. You can enter a word or a phrase such as the name of a medical condition or an intervention.
 Source: nlmdirector.nlm.nih.gov
Source: nlmdirector.nlm.nih.gov
2 days agoPhase 2. Janssen Research Development LLC First Posted. The NCT number in the IRB application must be entered in VIIB1b of the IRB application prior to IRB approval to show that the ClinicalTrialsgov record has been created. ClinicalTrialsgov Registration Data Elements 2 1. It is planned to recruit 111 subjects for 2 vaccines Eru-CoV-Vac or CoronaVac.
 Source: www1.udel.edu
Source: www1.udel.edu
An identifiers ID if any other than the organizations Unique Protocol Identification Number or the NCT number that is assigned to the clinical study. July 15 2021 Key Record Dates. The Centers for Medicare and Medicaid Services CMS issued a Transmittal requiring new mandatory reporting of the clinicaltrialsgov clinical trial number also known as NCT on all hospital and professional claims for related itemsservices. The NCT will be located under the Brief Title listed as the ClinicalTrialsgov Identifier. ClinicalTrialsgov - Notices of.
 Source: slidetodoc.com
Source: slidetodoc.com
This includes any unique clinical study identifiers assigned by other publicly available clinical trial registries. Use AND in uppercase to search for multiple terms. Date of Registration in Primary Registry Generated by system 3. August 4 2021 Last Verified. Search Tips and Examples.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title clinicaltrials gov search by nct number by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





